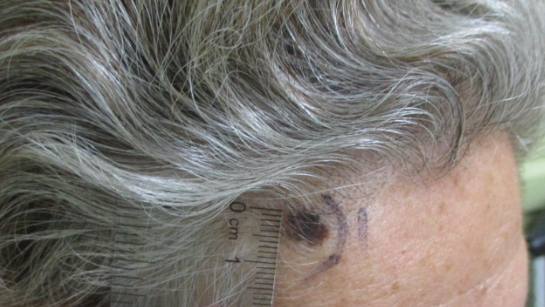
CORRECT DIAGNOSIS:
Malignant Melanoma
DISCUSSION:
The incidence of malignant melanoma in the United States has increased dramatically, rising over 320% since 1975. Histologic evaluation through skin biopsy remains the gold standard for diagnosis, while treatment recommendations vary based on several patient and lesion characteristics. Although malignant melanoma can affect any part of the body, sun-exposed areas are most commonly involved, as unprotected ultraviolet (UV) radiation exposure is the leading risk factor. Nevi may be categorized as benign, dysplastic (with varying degrees of atypia), or malignant. Stratifying atypia into categories such as mild, moderate, and severe can help assess the relative risk of progression from an atypical nevus to a malignant lesion. Individuals with multiple dysplastic nevi, a family history of first-degree relatives with melanoma, or moderate to severe dysplastic nevi are at an increased risk of developing malignant melanoma. Prospective studies have shown that conservative excision of dysplastic nevi with at least a 2 mm clinical margin results in no clinical recurrence over a median observed time of 16.9 months.
Early detection of atypical or suspicious nevi through regular skin evaluations in high-risk populations is critical to identifying lesions before they progress to malignant melanoma. Projections suggest that the incidence of malignant melanoma in the U.S. will peak between 2022 and 2026, emphasizing the urgency of timely treatment for dysplastic nevi. The overall risk of developing malignant melanoma in individuals with dysplastic nevi is significantly elevated, with some studies indicating a tenfold increase in risk compared to the general population. Dermatologists play a central role in the early diagnosis and treatment of this rapidly emerging health care issue, as they possess unique expertise in skin evaluation and screening of suspicious lesions. The annual costs of treating newly diagnosed melanoma at all stages across all age groups in the U.S. are estimated at $932.5 million. Reducing this burden could help redirect valuable resources back into the health care system.
A key challenge highlighted in this case is patient follow-up. Although clinicians can stress the urgency of treatment and the severity of the condition, loss to follow-up remains a persistent issue. Routine review of dysplastic nevus results, cross-referencing those results with medical records to identify pending treatments, and maintaining frequent communication with patients regarding follow-up appointments can help prevent patients from remaining untreated. Despite these efforts, our patient failed to return for treatment of her moderately dysplastic nevus for 37 months, resulting in the evolution of her lesion into invasive malignant melanoma.
TREATMENT:
The treatment for malignant melanoma depends on the stage of the disease, the location of the tumor, and patient-specific factors. Early-stage melanoma is often treated with surgical excision, which remains the cornerstone of treatment. The goal of excision is to remove the primary tumor with appropriate margins to reduce the risk of recurrence. For tumors with higher Breslow thickness, a wider excision margin of 1-2 cm may be recommended. If the melanoma has spread to nearby lymph nodes, a sentinel lymph node biopsy may be performed. For advanced melanoma, immunotherapy has emerged as a key treatment option. Immune checkpoint inhibitors, such as pembrolizumab and nivolumab, block the PD-1/PD-L1 pathway, allowing the immune system to better target and attack melanoma cells.
Additionally, for patients with BRAF-mutant melanoma, targeted therapy using BRAF inhibitors (e.g., vemurafenib) in combination with MEK inhibitors (e.g., trametinib) has shown promising results in improving patient outcomes. Although chemotherapy was once a standard treatment, it has largely been replaced by immunotherapy and targeted therapies but may still be used in specific cases, particularly for metastatic melanoma that has not responded to other treatments. Radiation therapy may also be used, particularly for melanoma that has metastasized to the brain or other areas where surgical resection is not feasible, or as an adjust following surgery if there is concern about incomplete resection or lymph node involvement. Finally, adjuvant therapy is recommended for patients with intermediate or high-risk melanoma following surgery to reduce the risk of recurrence. This approach, which includes immunotherapy or targeted therapy, has been shown to improve survival outcomes, especially for stage III melanoma patients.
In our case, the patient underwent excision of her malignant melanoma within one month of the re-biopsy. Dermatopathological analysis confirmed the diagnosis of malignant melanoma, with a maximum Breslow depth of 0.6 mm. Examination of the specimen margins showed complete clearance of the cutaneous portion of the melanoma. Given the patient’s high-risk malignancy, she was referred to Oncology for further evaluation to rule out any internal involvement.
This particular case is able to demonstrate the clinical course of a Dysplastic Nevus progressing to a Malignant Neoplasm in-vivo. The evolution of a lesion’s appearance on physical examination is a key feature of a high-risk lesion, however when evaluated at a single point in time, pathologic correlation is often required to stratify the risk of malignant transformation. Close follow-up and appropriate management can allow for the timely treatment of high-risk lesions before transforming into a malignancy.
REFERENCES:
Balch, C. M., Gershenwald, J. E., Soong, S. J., Thompson, J. F., Atkins, M. B., & Byrd, D. R. (2009). Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. Journal of Clinical Oncology, 27(36), 6199-6206. https://doi.org/10.1200/JCO.2009.23.4799. PMID: 19901007.
Chapman, P. B., Hauschild, A., Robert, C., Haanen, J. B., Ascierto, P. A., Larkin, J., & Dummer, R. (2011). Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New England Journal of Medicine, 364(26), 2507-2516. https://doi.org/10.1056/NEJMoa1103782. PMID: 21639808.
Eggermont, A. M., Chiarion-Sileni, V., Grob, J. J., Dalle, S., Dummer, R., Wolchok, J. D., & Robert, C. (2016). Adjuvant ipilimumab (anti-CTLA-4 antibody) versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A multicentre, double-blind, randomized phase 3 trial. The Lancet Oncology, 17(7), 848-858. https://doi.org/10.1016/S1470-2045(16)30106-3. PMID: 27237413.
Gershenwald, J. E., Scolyer, R. A., Hess, K. R., Long, G. V., Ross, M. I., & Puzanov, I. (2017). Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA: A Cancer Journal for Clinicians, 67(6), 472-485. https://doi.org/10.3322/caac.21391. PMID: 28909047.
Hauschild, A., Grob, J. J., Larkin, J., Schadendorf, D., Long, G. V., Dummer, R., & Ascierto, P. A. (2019). Dabrafenib plus trametinib in patients with BRAF V600E or V600K mutant advanced melanoma: A long-term follow-up analysis of overall survival and progression-free survival. The Lancet Oncology, 20(9), 1158-1167. https://doi.org/10.1016/S1470-2045(19)30327-5. PMID: 31472757.
Robert, C., Thomas, L., Bondarenko, I., O’Day, S. J., Weber, J., Garbe, C., & Lebbé, C. (2015). Pembrolizumab versus ipilimumab in advanced melanoma. New England Journal of Medicine, 372(26), 2521-2532. https://doi.org/10.1056/NEJMoa1503093. PMID: 25891173.
Saginala, K., Barsouk, A., Aluru, J. S., Rawla, P., & Barsouk, A. (2021). Epidemiology of melanoma. Med Sci (Basel), 9(4), 63. https://doi.org/10.3390/medsci9040063. PMID: 34698235.
Gandini, S., Sera, F., Cattaruzza, M. S., Pasquini, P., Abeni, D., Boyle, P., & Melchi, C. F. (2005). Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. European Journal of Cancer, 41(1), 28–44. https://doi.org/10.1016/j.ejca.2004.10.015. PMID: 15542313.
Terushkin, V., Ng, E., Stein, J. A., Katz, S., Cohen, D. E., Meehan, S., & Polsky, D. (2017). A prospective study evaluating the utility of a 2-mm biopsy margin for complete removal of histologically atypical (dysplastic) nevi. Journal of the American Academy of Dermatology, 77(6), 1096–1099. https://doi.org/10.1016/j.jaad.2017.07.016. PMID: 28803713.
Jeong, D. K., Bae, Y. C., Lee, S. J., Kim, H. S., & Choi, Y. J. (2019). A case of malignant melanoma after repeated recurrent dysplastic nevi. Archives of Craniofacial Surgery, 20(4), 260–264. https://doi.org/10.7181/acfs.2019.00283. PMID: 31462019.
Guy, G. P. Jr., Ekwueme, D. U., Tangka, F. K., & Richardson, L. C. (2012). Melanoma treatment costs: A systematic review of the literature, 1990-2011. American Journal of Preventive Medicine, 43(5), 537–545. https://doi.org/10.1016/j.amepre.2012.07.031. PMID: 23079178.