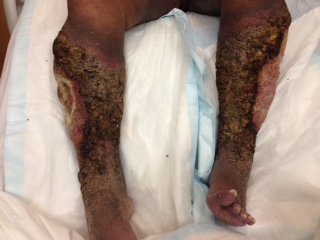
CORRECT DIAGNOSIS:
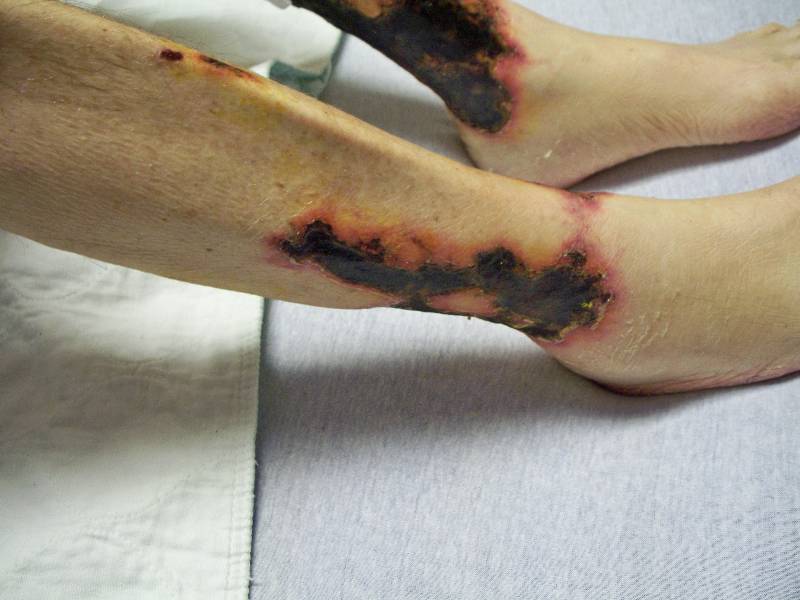
Calciphylaxis
DISCUSSION:
Calciphylaxis (synonyms: calcific uremic arteriolopathy, calcifying panniculitis, uremic gangrene syndrome, vascular calcification-cutaneous syndrome, and calcific azotemic arteriopathy) is a rare, usually fatal, disorder of progressive vascular calcification, with subsequent ischemic cutaneous and soft tissue necrosis. Often, this phenomenon is associated with end-stage renal disease (ESRD), and is usually seen in hemodialysis (HD) and renal transplant patients. However, a number of patients have been shown to have minimal or no renal disease. The pathophysiology of calciphylaxis may be considered the cutaneous equivalent of myocardial infarction. Medial calcification and subintimal fibrosis of arterioles results in arteriolar stenosis, which is then followed by thrombotic occlusion and cutaneous necrosis.
Clinically, reticulated, violaceous, mottled patches appear initially. These later evolve into painful, purpuric, subcutaneous plaques and nodules, accompanied by livedo reticularis. Necrotic ulcers with black eschars ensue as the disease progresses. These lesions often appear on the abdomen, thighs, and extremities; however, necrosis may also involve other sites, such as the digits, penis, tongue, or breast. The diagnosis of calciphylaxis is determined via skin biopsy. Histologic features include medial calcification of small and medium-sized blood vessel walls (pannicular small arteries, arterioles, and venules) with or without intimal fibroplasia, intravascular calcium deposition, and luminal thrombosis of dermal and subcutaneous vessels. Epidermal, dermal, and pannicular necrosis is also seen. It is noteworthy that a deep incisional biopsy is likely to provide a better histological yield. Special stains include the Von Kossa stain to demonstrate calcium deposits, as well as other stains to reveal the degeneration of elastic fibers. Direct immunofluorescence is negative for vascular deposition of immunoreactants.
Additionally, there are several laboratory values that are suggestive of the disease process. Patients with significant renal disease often exhibit a high serum calcium-phosphorous product, in which value more than 60-70 mg/dL indicates great risk for calciphylaxis. However, a lack of calcium-phosphorous serum elevations has been reported in many patients, especially in those with minimal or no renal disease. Parathyroid hormone is also classically elevated. Metabolic and electrolyte abnormalities are found in the majority of patients, often with a decreased serum albumin and increased total alkaline phosphatase. Additionally, increased erythrocyte sedimentation rate and hypercoagulable states are noted to increase a patient’s risk of calciphylaxis, in which decreased levels of proteins C and S have been detected. Lupus antiphospholipid antibody and anticoagulant panels are often both positive as well.
Upon review of our patient’s laboratory values, both BUN and creatinine were elevated at 45 mg/dL and 4.3 mg/dL, respectively, due to his significant renal disease. However, contrary to what is often found with calciphylaxis associated with ESRD and HD, the case study exhibited normal calcium of 8.8 mg/dL, normal ionized calcium of 1.3 mmol/L, a slightly elevated phosphorous of 5.3 mg/dL, along with a normal calcium-phosphorus product of 46.64 mg/dL. Additionally, the parathyroid hormone intact (PTHI) was normal at 15.6 pg/mL. Thus, while the typical case of calciphylaxis involves renal disease characterized by these abnormalities, renal disease with normal calcium-phosphorus and parathyroid hormone levels has also been reported with calciphylaxis, as in the case of our patient. Furthermore, albumin was decreased at 1.1 mg/dL and alkaline phosphatase was elevated at 286 units/L, both of which were supportive of calciphylaxis. The presence of the disease process was further substantiated with positivities of the lupus antiphospholipid antibody and anticoagulant panels, characterized by a positive lupus antiphospholipid antibody > 8 seconds, an elevated APTT 1.5 hour at 46 seconds, and an elevated APTT 1:1 saline at 57 seconds. The remainder of the lupus anticoagulant and antiphospholipid panels were, however, negative with a normal LA 1 (DRVVT) and LA2 (DRVVT). The patient was also shown to be in a hypercoagulable state, which is a risk factor for calciphylaxis, in which he had a decreased protein C antigen at 43%. However, protein S, both free and total, were normal at 76% and 69%, respectively. IgG was elevated at 1730 mg/dL, whereas IgM was normal at 75 mg/dL. ANA, rheumatoid factor, and cryoglobulins were all negative.
In summary, although the laboratory abnormalities described above are good markers for calciphylaxis, they tend to vary from patient to patient, and so can make the identification of calciphylaxis all the more challenging.
TREATMENT:
To date, many treatment modalities have been utilized; yet there remains a high degree of outcome inconsistency and variability, in which most attempts are unsuccessful. Consequently, calciphylaxis is a deadly disease with a mortality rate of up to 80%. Therefore, aggressive treatment is essential. Monitoring the patient’s metabolic environment is of utmost importance. Hyperphosphatemia must be controlled with non-calcium-containing phosphate binders. A phosphorous-restricted diet should be introduced, and calcium/ vitamin D supplementation discontinued. Low serum albumin should also be treated. Because most patient deaths occur from sepsis, aggressive wound care and debridement of necrotic tissue, monitoring for infection, and prophylactic antibiotics should all be initiated. Intravenous sodium thiosulfate, a calcium chelator, has emerged as another first-line therapy. For patients that do not exhibit ulcerations, oral prednisone has appeared helpful. Second-line therapies that can be attempted are parathyroidectomy (for patients with elevated parathyroid hormone), hyperbaric oxygen therapy to the necrotic wounds, low-dose tissue plasminogen activator (thrombolytic) for treatment of the vascular occlusion, as well as zero- or low-calcium dialysate, Cinacalcet (calcimimetic), and intravenous pamidronate (bisphosphonate) for normalization of calcium levels. Lastly, the treatment of calciphylaxis should be multidisciplinary with the involvement of multiple specialties.
Sodium thiosulfate, the main course of treatment which was attempted in our patient, had to be discontinued within 24 hours of its initiation, due to the patient exhibiting a drug reaction, characterized by urticaria, angioedema, and stridor requiring respiratory support. Low calcium dialysis and phosphate binders were given via HD. Due to the critically high heparin pf4 antibody, Argatroban was given for possible heparin-induced necrosis, although this was felt unlikely, due to the clinical presentation and non-specific nature of this antibody. Intravenous antibiotics were also initiated, prophylactically for infection. Dressing changes were applied twice daily, with 0.9% NS solution followed by Mupirocin 2% cream and trolamine salicylate cream, to all areas directly within the wounds, accompanied by Triamcinolone 0.1% cream BID to the peri-wound. Other treatments, such as parathyroidectomy, tissue plasminogen activator, and surgical debridement, were all considered; however, the patient has not deemed a candidate for any of these, due to his critically ill state. Pamidronate, another possible treatment modality, was not deemed beneficial, due to the patient already having normal calcium and only slightly elevated phosphate level. Hyperbaric oxygen was also recommended to the wound care team. The necrotic skin lesions improved only minimally, and the patient ultimately requested a transfer to the Hospice unit, where he later passed away.
REFERENCES:
Dauden, E., & Onate, M.-J. (2008). Calciphylaxis. Dermatologic Clinics, 26(4), 557-568. PMID: 18864144
Robinson-Bostom, L., & DiGiovanna, J. (2002). Cutaneous manifestations of end-stage renal disease. Journal of the American Academy of Dermatology, 46(6), 975-986. PMID: 12084912
Norris, B., Vaysman, V., et al. (2005). Bone scintigraphy of calciphylaxis: A syndrome of vascular calcification and skin necrosis. Clinical Nuclear Medicine, 30(12), 725-727. PMID: 16385340
Hussein, M.-R., Ali, H., et al. (2009). Calciphylaxis cutis: A case report and review of literature. Experimental and Molecular Pathology, 86(2), 134-135. PMID: 19195996
Lebwohl, M., et al. (2010). Treatment of Skin Disease: Comprehensive Therapeutic Strategies (3rd ed., pp. 117-119).
Weenig, R. (2008). Hans Selye to nuclear factor k-B. Journal of the American Academy of Dermatology, 58(3), 458-471. PMID: 18378006
Weenig, R., Sewell, L., et al. (2007). Calciphylaxis: Natural history, risk factors, and outcomes. Journal of the American Academy of Dermatology, 56(4), 569-579. PMID: 17234481
Arseculeratne, G., Evans, A., et al. (2006). Calciphylaxis: A topical review. European Academy of Dermatology & Venereology, 20(4), 493-502. PMID: 17040196
Joukhadar, R., & Bright, T. (2009). Calciphylaxis in primary hyperparathyroidism: A case report and brief review. Southern Medical Journal, 102(4), 318-321. PMID: 19337166
Robinson, M., Augustine, J., et al. (2007). Cinacalcet for the treatment of calciphylaxis. Archives of Dermatology, 143(2), 152-154. PMID: 17224445
Chavel, S., Taraszka, K., et al. (2004). Calciphylaxis associated with acute, reversible renal failure in the setting of alcoholic cirrhosis. Journal of the American Academy of Dermatology, 50(1), S125-S128. PMID: 14712780
Nigwekar, S., Wolf, M., et al. (2008). Calciphylaxis from nonuremic causes: A systemic review. Clinical Journal of the American Society of Nephrology, 3(4), 1139-1143. PMID: 18256081
Weenig, R., Gibson, L., et al. (2007). The role of the hospital dermatologist in calciphylaxis and nephrogenic systemic fibrosis. Seminars in Cutaneous Medicine and Surgery, 26(3), 163-167. PMID: 17966801
Hanafusa, T., Yamaguchi, Y., et al. (2007). Intractable wounds caused by arteriosclerosis obliterans with end-stage renal disease treated by aggressive debridement and epidermal grafting. Journal of the American Academy of Dermatology, 57(3), 322-326. PMID: 17698194
Meissner, M., Gille, J., et al. (2006). Calciphylaxis: No therapeutic concepts for a poorly understood syndrome? Journal der Deutschen Dermatologischen Gesellschaft, 4(12), 1037-1044. PMID: 17196164
Baker, B., Fitzgibbons, C., et al. (2007). Calciphylaxis responding to sodium thiosulfate therapy. Archives of Dermatology, 143(2), 269-270. PMID: 17224445
Hayden, M., Goldsmith, D., et al. (2008). Calciphylaxis: Calcific uremic arteriolopathy and the emerging role of sodium thiosulfate. International Urology & Nephrology, 40(2), 443-451. PMID: 17978836
Ackerman, F., Levy, A., et al. (2007). Sodium thiosulfate as first-line treatment for calciphylaxis. Archives of Dermatology, 143(11), 1336-1338. PMID: 18056193
Rogers, N., Teubner, D., et al. (2007). Calcific uremic arteriolopathy: Advances in pathogenesis and treatment. Seminars in Dialysis, 20(2), 150-157. PMID: 17302912
Hanafusa, T., Yamaguchi, Y., et al. (2007). Intractable wounds caused by calcific uremic arteriopathy treated with bisphosphonates. Journal of the American Academy of Dermatology, 57(6), 1021-1025. PMID: 17449071
Hafner, J., Keusch, G., et al. (1995). Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): A complication of chronic renal failure and benefit from parathyroidectomy. Journal of the American Academy of Dermatology, 33(6), 954-962. PMID: 7500970
Robinson, M., Augustine, J., et al. (2007). Cinacalcet for the treatment of calciphylaxis. Archives of Dermatology, 143(2), 152-154. PMID: 17224445