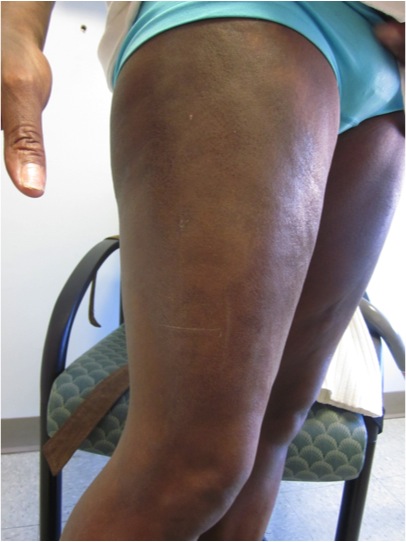
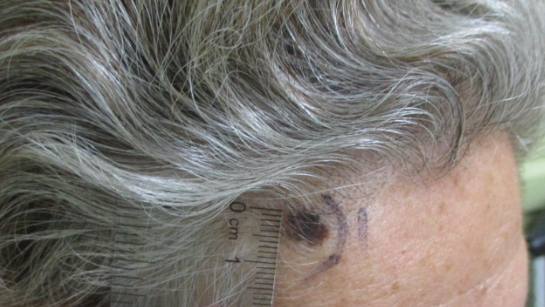
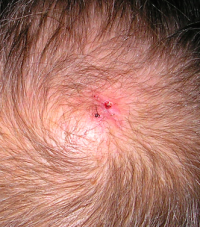
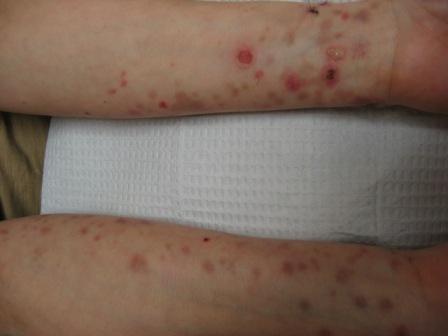
CORRECT DIAGNOSIS:
Scleroderma (SSc)
DISCUSSION:
Systemic sclerosis (SSc), also known as scleroderma, is a complex disorder characterized by vascular alterations, autoantibodies against various cellular antigens, and extensive fibrosis. [1] Although at present there is no single unifying hypothesis to explain all aspects of the disease, evidence has shown the primary insult to be directed against blood vessels, causing endothelial cell injury. Chronic inflammatory cell infiltration and subsequent cytokine production, combined with oxidative damage and cell injury results in the development of tissue fibrosis. [2]
The disease is divided into two conventionally used subsets, despite microarrays revealing more subsets than previously appreciated. Autoantibodies are used to help classify subtypes and convey both diagnostic and prognostic information. The most prevalent autoantibodies associated with SSc are anticentromere antibodies, anti-topoisomerase I (or anti-Scl70) antibodies, and anti-RNA polymerase III antibodies. They are all mutually exclusive and tend not to overlap with autoantibodies from other autoimmune diseases. [3]
The two main subsets of SSc are limited cutaneous (lcSSc) and diffuse cutaneous (dcSSc). The limited cutaneous form is characterized by an initial onset of Raynaud’s phenomenon in the early stages of the disease. [3] Raynaud’s phenomenon is a reversible spasm of the small arteries and arterioles in the fingers and toes. [4] Several years after the appearance of Raynaud’s, the cutaneous fibrosis component manifests, but only as thickening of the skin of fingers and hands. CREST syndrome represents a subset of lcSSc, defined by calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasias. Seventy percent of lcSSc patients have anticentromere antibodies, which confer an increased risk of pulmonary artery hypertension (PAH). [3] The anticentromere antibodies associated with lcSSc are typically more common in Caucasians. [5] Diffuse cutaneous systemic sclerosis presents initially with symmetric finger and hand swelling. It later generalizes to the forearms, arms, face, trunk, and lower extremities. In general, the onset of edema in dcSSc occurs shortly after the first episode of Raynaud’s. The edema becomes a firm, bound-down induration, with fibrosis leading to deformities of the digits such as fixed flexion contractures of the proximal interphalangeal joints. Twenty percent of dcSSc patients have anti-topoisomerase I antibodies, which confer a worse prognosis with increased pulmonary disease and increased mortality.[3] The presence of anti-topoisomerase I antibodies are found to be more common in African Americans. [5] Another twenty percent of dcSSc patients have anti-RNA polymerase III antibodies, which confer an increased risk of scleroderma renal crisis (SRC) .[3]
Environment and Genetics
The extremely complex pathogenesis of SSc generally involves abnormalities of both the vascular and immune systems, which ultimately lead to fibrosis of end organs. Several etiologies are suspected to be linked to the pathogenesis of SSc. Environmental factors such as occupational and industrial exposure to vinyl chloride, silica dust, and organic solvents, as well as drugs such as bleomycin, pentazocine, and cocaine have all been implicated as potential causes of disease. [3] Gadolinium-based MRI contrast has been reportedly linked to nephrogenic systemic fibrosis, a condition of renal dysfunction with thickening and hardening of the skin. Nephrogenic systemic fibrosis has recently been demonstrated to have increased expression of transforming growth factor-beta (TGFβ), which is one of the most well-described growth factors involved in the pathogenesis of SSc.[6]
Infectious agents have also been shown to play a role in the immunopathogenesis of SSc. Viruses such as cytomegalovirus (CMV) and parvovirus B19 have been linked to SSc development. It is thought that molecular mimicry occurs, whereby antibodies that recognize viral antigens cross-react with endothelial cells to induce apoptosis. [3] More recent studies have expanded the link between infectious agents and SSc to include Epstein-Barr virus (EBV), Hepatitis B virus (HBV) and Toxoplasma gondii, shown by demonstrating high titers of antibodies against these infectious antigens in patients with scleroderma. [7]
There is conflicting evidence to support the contribution of genetics to SSc pathogenesis. The largest twin study to date showed a low concordance rate of SSc among identical twins, suggesting a low heritability component in the development of the disease. [3] However, despite this seemingly low rate, it is still approximately 300 times higher than the frequency expected by chance alone. [5] The Choctaw Indians of Oklahoma reportedly had the highest worldwide prevalence rates of SSc, and a unique human leukocyte antigen (HLA) haplotype that may confer a genetic risk. Furthermore, studies of a Korean cohort and two Caucasian cohorts showed susceptibility at two HLA loci. Several genetic polymorphisms have been identified to confer susceptibility to SSc, however, they have failed to be confirmed in follow-up studies. 3,8 Overall, a specific genetic mode of inheritance of SSc remains to be elucidated. Despite the lack of a clear genetic inheritance, gene expression in scleroderma skin is distinct from that of healthy controls, with over 2,000 genes differentially expressed. This was demonstrated in studies showing both lesional and nonlesional biopsies of SSc patients to exhibit nearly identical patterns of gene expression; ones which are distinct from healthy controls .[3] Based on the currently available data, it is likely that genetic factors provide a susceptible environment that predisposes individuals to the development and progression of SSc.
A highly controversial theory behind the development of SSc is the concept of microchimerism. It has been hypothesized that allogenic fetal and maternal cells that cross the placenta during gestation may contribute to disease pathogenesis. HLA compatibility allows for fetal or maternal cells to cross the placenta and persist in the bloodstream. The theory entails that these engrafted fetal cells become activated by some external trigger, and mount a graft-vs-host response on the mother or future offspring, thus resulting in SSc disease manifestation. [5] Another concept linked to the development of SSc is alterations in collagen transcription genes. In SSc, fibroblasts have an increased expression of genes involved in the production and deposition of type I collagen. Type I collagen is the most prevalent protein found in patients with SSc, and is thought to be responsible for the most severe clinical symptoms. The disease state of SSc can be distinguished from normal wound healing by the autonomous and persistent upregulation of collagen gene expression. [5]
Immunologic alterations
Immune dysregulation, and increased circulating levels of cytokines, also play a role in the development of SSc. [9] The humoral immune system has gained recent attention, as one of the most common manifestations of SSc is the presence of specific autoantibodies. Some of them have been described as exclusively associated with SSc, while others are associated with various clinical manifestations of the disease such as pulmonary or other organ involvement. [5] Despite the well-described presence of autoantibodies, the jury remains out on whether or not they are directly involved in actual disease development and progression. Although microarray analyses of skin biopsies have shown conflicting results, a role of B cell autoantibodies in the pathogenesis of SSc is implicated. Lung tissue histology from SSc patients has demonstrated lymphoid aggregates of B cells, suggesting their involvement in lung disease. Investigators have also shown overexpression of activation markers for memory B cells in SSc patients compared to controls. [3]
The cellular immune system, and activity of T helper (Th) cells, is tightly linked to SSc. Studies have shown a predominance of Th2 peripheral blood cells in SSc patients, as compared to Th1 cells. Of note, these immune cells are most pronounced in those with interstitial lung disease, corresponding to a decreased forced vital capacity. Furthermore, increased IL-13 correlates with a greater degree of fibrosis, particularly in those with diffuse type SSc. It is suspected that IL-13 contributes to fibrosis through TGFβ induction by macrophages. [3] TH2 cytokines such as IL-4, IL-5, IL-13 and IL-21 can also act independently of the TGFβ signaling pathway in stimulating fibroblast proliferation and collagen deposition. [10] Several reports have implicated the role of TH1 cells in SSc, specifically from observations of increased interferon (IFN) gene-expression signatures. There was found to be increased IFN- mRNA in vascular and perivascular cells. This data suggests a local activation of leukocytes within the vasculature. It has also been shown that anti-topoisomerase I antibodies induce significantly elevated IFN-alpha levels, while anticentromere antibodies appear to have a negative regulatory impact on IFN-alpha. Lung biopsies have linked increased induction of IFN-alpha with greater tissue injury. This provides partial evidence for the diverse phenotypes seen in limited compared to diffuse SSc subsets, by demonstrating increased degrees of IFN response leading to more severe lung fibrosis. [3] Numerous chemokines have been implicated in the development of SSc, but chemokine CCL2 has received the most attention. Both the skin and serum of SSc patients have shown elevated levels of CCL2, and in some cases, corresponding to major organ involvement such as pulmonary fibrosis. It has been suggested that an autocrine loop of CCL2 and its receptor, CCR2, may be contributing to the early stages of fibrosis. [3]
Fibrosis
Fibrosis results from chronic inflammation, defined as an immune response that persists for several months, in which tissue remodeling and repair processes occur simultaneously. This is in contrast to acute inflammation, characterized by rapidly resolving vascular changes, edema, and a neutrophilic response. Chronic fibrosis usually results from a common persistent irritant that sustains the production of growth factors, proteolytic enzymes, angiogenic factors, and fibrogenic cytokines. The irritants leading to tissue damage include infections, autoimmune reactions, toxins, radiation, and mechanical injury. These irritating stimuli result in the deposition of connective tissue components that progressively remodel and destroy normal tissue architecture. [10] In SSc, the previously described etiologic factors, as well as endothelial cell damage and vascular alterations which will be detailed later, represent the irritating stimuli that result in fibrosis.
The process of tissue repair typically involves a regenerative phase and a fibrosis phase. The regenerative phase has no lasting evidence of damage, as injured cells become replaced by cells of the same type. In the fibrosis phase or fibroplasia, normal parenchymal tissue becomes replaced by connective tissue. [10] Under normal conditions, fibroblasts produce little extracellular matrix (ECM) components. [3] However, the generally beneficially repair process becomes pathogenic when normal tissue is replaced by permanent fibrotic scar tissue from the substantial deposition of ECM. [10] The myofibroblast is a modified fibroblast, which exhibits features of smooth muscle cells that are critical for tissue contraction and remodeling during the wound healing process. [3] Fibrosis occurs when the synthesis of new collagen by myofibroblasts exceeds the rate of degradation, leading to an overall increase in collagen. [10] This unregulated wound healing due to activation and differentiation of fibroblasts into collagen and ECM producing myofibroblasts is a highly complex process. It is partially attributable to local tissue injury causing the release of cytokines and growth factors. [3] In SSc, this remodeling and fibrosis ultimately lead to organ failure and death.
Fibroblasts obtained from lesional skin or fibrotic lungs in SSc patients were shown to have constitutively activated myofibroblast-like phenotypes, characterized by enhanced ECM synthesis with the secretion of cytokines and chemokines and increased expression of cell surface receptors. [10] Various mechanisms are thought to be responsible for regulating myofibroblast activity. The exact origin of myofibroblasts in SSc has yet to be definitively established, however, a few potential sources have been described. They are thought to be derived from local fibroblasts, transdifferentiation of epithelial and endothelial cells known as epithelial- or endothelial-to-mesenchymal transition, bone marrow stem cell-derived fibrocytes or smooth muscle-like pericytes found in vascular walls. [3] It is thought that the SSc fibroblast phenotype is maintained by an autocrine TGFβ signal, since these fibroblast characteristics are reproducible in normal human fibroblasts following TGFβ stimulation. The cytokine TGFβ is the most extensively studied regulator of the ECM, and tightly linked to the development of fibrosis in disease. [10] TGFβ induces fibrosis via stimulation of ECM synthesis by fibroblasts and myofibroblasts. [3] It also acts by decreasing the synthesis of collagen degrading metalloproteinases, and stimulating the production of metalloproteinase inhibitors. [5] TGFβ is secreted by numerous cell types including fibroblasts, myofibroblasts, T cells, monocytes, macrophages, and platelets, [3] though circulating monocytes and macrophages are the primary sources of TGFβ. Regulation occurs at the level of TGFβ secretion and activation, as opposed to translational expression. TGFβ is stored in its latent form, and activated by various proteases. [10] TGFβ signal transduction is highly intricate, involving multiple receptors and signaling intermediates. It appears that TGFβ sensitizes fibroblasts and maintains them in a persistently activated state via an autocrine mechanism loop leading to further TGFβ production. [5]
Critical to the pathogenesis of SSc, is the signaling cascade involving the SMAD family of proteins. Activated TGFβ signals phosphorylation of SMAD protein intermediates via transmembrane receptors, to modulate the transcription of collagen. Once activated, TGFβ binds to its receptor, which then becomes phosphorylated. The phosphorylated receptor subsequently signals phosphorylation of SMAD2 and SMAD3, thereby activating them. Activated SMAD2 and SMAD3 then form a complex with SMAD4, which allows for translocation of the entire complex into the nucleus. Once within the nucleus, intranuclear proteins aid the complex in binding directly to promoter regions of genes responsible for collagen production. [5] Dysregulation of the SMAD and non-SMAD downstream pathways of TGFβ activation contributes to the abnormal fibrogenic response seen in SSc. Of note, SMAD7 is an inhibitory SMAD which can bind to the TGFβ receptor complex early in the cascade, and prevent phosphorylation of SMAD2 and SMAD3. Recent studies have described substantially decreased SMAD7 levels in fibroblasts and skin of SSc patients. [5]
Evidence exists to support the role of connective tissue growth factor (CTGF) and endothelin-1 (ET-1) in persistent fibroblast activation due to the autocrine TGFβ loop, as these factors are induced by TGFβ. [3] CTGF is involved in angiogenesis and the structural organization of connective tissue. [5] It also exerts a profibrotic effect via the upregulation of collagen, fibronectin and integrin, and stimulation of fibroblast proliferation. Although the exact pathogenesis of CTGF is not fully understood, it is postulated to represent a downstream mediator of TGFβ. ET-1 is a potent vasoconstrictor, with additional pro-fibrotic properties.
Vascular and endothelial aberrations
Chronic fibrosis in SSc is tightly linked to vascular disruption which precedes the fibrotic changes. Vascular disease results from damage to the endothelial cell layer of the microvasculature. This injury leads to over-expression of adhesion molecules, leukocyte and pericyte proliferation, platelet adhesion and activation, and the influx of perivascular infiltrate. Myofibroblasts in the endothelium create a vasculopathy of intimal thickening, fibrosis, and marked luminal narrowing, with fairly normal media and smooth muscle hypertrophy. This results in compromised regional blood flow, with tissue ischemia and fibrosis that ultimately lead to organ dysfunction. [2]
Electron microscopy of skin biopsies from SSc patients show signs of endothelial injury, apoptosis, and perivascular edema. In SSc there exists endothelial cell injury and capillary destruction, accompanied by a perivascular reaction involving immune cells and fibroblasts. [2] Increased vascular permeability allows mononuclear cell transmigration into the perivascular space, resulting in edema. This vascular-cellular interaction precedes the later tissue fibrosis, occurring as vessels loose elasticity to become fibrotic and occluded. [3]
When vascular endothelium is disrupted, there is a downstream alteration of “biomarkers”, which are certain molecules having increased production or impaired release. This imbalance of regulatory factors alters local blood flow, and contributes to the vascular instability of SSc. Disrupted endothelial cells result in increased coagulation, platelet activation, and release of adhesion molecules and proangiogenic factors. [2] The highly upregulated proangiogenic factors such as platelet-derived growth factor (PDGF), TGFβ, ET-1 and chemokine CCL2 are activators of smooth muscle cells and stromal fibroblasts, thus contributing to proliferative vasculopathy and fibrosis. [3] The platelet products PDGF and TGFβ also increase production and deposition of ECM. Skin biopsies have demonstrated increased circulating levels of the biomarker von Willebrand factor, shown to leak into the perivascular space of scleroderma patients. There is a further increase in the potent vasoconstrictor ET-1, which acts on vascular smooth muscle cells to potentially induce myofibroblast expression, 2 along with exhibiting pro-fibrotic properties via activation of TGFβ, as previously mentioned.
Despite an increase in the aforementioned proangiogenic factors, in vitro studies paradoxically show defective angiogenesis in SSc patients, evident by decreased capillary density and low expression of molecules that facilitate the action of vascular endothelial growth factor (VEGF). Some studies have led to the implication of a soluble inhibitor of angiogenesis. During the early, acute phase of SSc, proangiogenic factors like VEGF are released, however, antiangiogenic proteolytic enzymes also become activated. More severe disease is noted to have elevated levels of endostatin, an angiogenesis inhibitor derived from collagen. Stabilization of proangiogenic factors becomes blocked by the concurrent up-regulation of these angiogenesis inhibitors. This imbalance of proangiogenic and antiangiogenic factors favors decreased ability for new vessel formation. [2]
Systemic sclerosis is also characterized by impaired vasculogenesis or vascular repair. Endothelial progenitor cells (EPCs) are mononuclear cells produced in the bone marrow, which travel to sites of vascular injury and ischemia to mediate vasculogenesis. [3] Advanced stages of SSc have shown patients with significantly fewer and functionally impaired EPCs. [11] When bone marrow mesenchymal cells are defective, SSc patients are unable to repair or replace endothelial cells after injury. Studies have shown that administration of statins (HMG-CoA reductase inhibitors), which increase progenitor cells necessary for vasculogenesis, result in increased circulating endothelial cells. Further research is needed to confirm this finding. [2]
Nitric oxide (NO) is a potent vasodilator, which also inhibits platelet aggregation and reduces endothelial cell activation by cytokines. Angiotensin II (ANG II) is a vasoconstrictor which regulates cell growth, inflammation, and vascular fibrosis by increasing activation of TGFβ and CTGF. In SSc patients, the dysfunctional vascular tone is attributed to both an intrinsic defect in NO production in endothelial cells, and an increased level of ANG II. Raynaud’s phenomenon, characterized by vasospasm of digital arteries and cutaneous vessels, is partly influenced by impaired vasodilation from a deficiency of the vasodilatory neuropeptides substance P and calcitonin gene-related peptide (CGRP). [2]
The exact pathogenesis leading to endothelial cell injury in SSc is not entirely elucidated, but several possible causes have been proposed. Data have suggested that activated cytolytic T cells cause endothelial cell injury via the release of granzyme proteolytic enzymes. [2] The role of anti-endothelial cell antibodies (AECAs) has also been proposed, and it is suggested that the presence of AECAs may correlate with EPC death by apoptosis. [11] Systemic sclerosis patients possess IgG antibodies that react with endothelial cells. It remains unclear whether these antibodies serve as a primary mechanism of endothelial damage, or represent a secondary consequence of such injury. [3] Nonetheless, the presence of these antibodies correlates with more severe clinical vascular disease including digital ischemia, abnormal nail fold capillaries, PAH, and microvascular disease in pulmonary fibrosis. [2] As previously described, the viral and bacterial infection might play a role in endothelial apoptosis, as there are increased anti-CMV antibodies found in SSc patients. Furthermore, activation of the cellular and the humoral immune system is thought to lead to endothelial injury. Studies have shown defective complement due to loss of protective molecules, representing either a sign of vascular injury itself or a contributing factor to the damage via complement activation. [2] Pericytes are cells, which support endothelial cell functions in arterioles, capillaries, and venules. In scleroderma, pericytes are hyperplastic, and when activated express PDGF and interact with fibroblasts in the early stages of the disease. It is possible that these pericyte-fibroblast interactions contribute to the vascular disruption seen in SSc. [2]
Several studies have shown decreased serum antioxidant levels and increased markers of oxidative damage in scleroderma patients. [3] One cohort of SSc patients were shown to have deficiencies of ascorbic acid and selenium, [12] while another showed reduced concentrations of alpha-tocophorol (vitamin E) and carotene. [13] Signs of lipid peroxidation were demonstrated using the clinical marker F2 isoprostanes. These F2 isoprostanes represent a family of compounds generated from arachidonic acid via a free radical-catalyzed mechanism. Elevated levels of urinary concentrations of F2 isoprostanes have been shown in SSc when compared to healthy control subjects. [14] Despite clear evidence of oxidative damage, the primary insult leading to reactive oxygen species (ROS) generation remains to be elucidated. [3] It is known that vasoconstriction leading to decreased tissue perfusion and hypoxia causes cell injury and alterations in function. [2] Tissue hypoxia induces ECM genes, which mediate fibrosis. Ischemia leads to the release of free radicals into the vascular cells from macrophages, endothelial cells, and vascular smooth muscle cells. Lipoproteins represent an additional contribution to oxidative damage. Studies show that SSc patients have increased levels of oxidized lipoproteins such as LDL, which has innate properties of enhancing smooth muscle cell proliferation and activating endothelial cells. [2] The oxidative stress caused by these reactive oxygen species mediates fibrosis via direct activation of fibroblasts and endothelial cells, and by releasing the pro-fibrotic cytokines TGFβ and PDGF. Perivascular cells in SSc are also shown to have increased PDGF receptors. Continuous oxidative stress from repeated ischemia-reperfusion injury might lead to the expression of neoantigens that perpetuate an autoimmune response. [2] The question remains, however, whether ROS initiate the vascular damage and instability leading to Raynaud’s phenomenon, or if the ischemia-reperfusion injury from vasospasm leads to ROS generation.
The role of antiphospholipid antibodies in SSc is unclear, although they are suggested to be involved in macrovascular disease, as their presence has been shown associated with digital ischemia. [2] Alterations in coagulation are thought to be attributable to endothelial cell injury as well. The endothelium is responsible for maintaining an antithrombotic lining, with tight regulation of the coagulation process. [3] In patients with SSc, elevated fibrinogen levels and defective tissue plasminogen activator (tPA) release alters the balance of intravascular coagulation and fibrinolysis to favor coagulation. Tissue plasminogen activator is necessary to cleave plasminogen into plasmin, which is necessary for mediating fibrinolysis. A deficiency of tPA alters the fibrinolytic pathway, thus predisposing SSc patients to fibrin deposition and vascular obstruction due to thrombosis. [2]
Clinical manifestations
Within the skin of SSc patients, there is increased collagen, abnormal vasculature, and an inflammatory infiltrate. The collagen consists of a homogenous, hyalinized pattern extending from the papillary dermis to the subcutis. This increased collagen ultimately replaces subcutaneous fat and eccrine sweat glands, which have atrophied. [3] Direct immunofluorescence studies are usually negative for immunoglobulin deposition at the dermo-epidermal junction and within the microvasculature, distinguishing SSc from other autoimmune connective tissue disorders such as systemic lupus erythematosus (SLE). [3] Although SSc has long been considered a disease of tissue fibrosis, the associated endothelial cell injury and vascular disruption play a fundamental role in tissue damage. The clinical consequences of vascular disease are not limited to cutaneous vessels but involve vasculature of the extremities and multiple organs. [2] The widespread effects of vascular disease and tissue fibrosis can lead to internal organ dysfunction, a significant cause of morbidity and mortality among SSc patients.
Raynaud’s phenomenon is caused by disease of the thermoregulatory vessels in the skin and small to medium vessels of the peripheral arterial system of limbs. The normal vasospastic response to environmental decreases in temperature is exaggerated, resulting in color changes of the skin (pallor, cyanosis, or hyperemia). The attacks are typically symmetrical and resolve 15-20 min after re-warming. Systemic sclerosis patients experience frequent ischemic events that can result in digital ulcers or amputation. The nail fold capillaries are also impacted by the microvascular disease in SSc. The damage is characterized by cutaneous capillary structural alterations and decreased density and blood flow. Nailfold capillaries demonstrate enlarged capillary loops surrounded by avascular areas. Telangiectasias of the face, hands, fingers, and mucous membranes are common among scleroderma patients as well. While more likely to occur with limited SSc subtypes, they are present in later stages of all subtypes. Telangiectasia lesions consist of vasodilated postcapillary venules, without inflammation or neovascularization. They are thought to arise from a failed or aberrant effort of angiogenesis. [2]
As the clinical disease is not limited to the skin, there are widespread systemic effects occurring in SSc patients, often entailing a macrovascular disease of arteries. The interstitial pulmonary disease represents the most common cause of death in SSc patients. This life-threatening pulmonary pathology can result, either by nonspecific interstitial pneumonia leading to pulmonary fibrosis, or pulmonary artery hypertension (PAH) due to an obliterative vasculopathy. Pulmonary fibrosis begins as patchy inflammatory infiltrates of lymphocytes, eosinophils, and macrophages within alveolar walls, which then progresses to fibrosis as the alveolar septae thicken. Pulmonary artery hypertension occurs by large artery intimal thickening and proliferation. [3] Scleroderma renal crisis (SRC) is characterized by accelerated hypertension and acute renal failure. It is caused by reversible vasospasm of arcuate and interlobular renal arteries, as well as duplication of the elastic lamina and intimal proliferation with luminal occlusion. [3] Cardiac dysfunction results from both occlusive vascular disease and intermittent vasospasm known as “intramyocardial Raynaud’s phenomenon.” This leads to ischemic events and contraction band necrosis from reperfusion injury. [2] Fibrosis of the cardiac conduction system can also lead to arrhythmias. [3] Loss of bowel smooth muscle and tissue fibrosis from mouth to anus leads to gastrointestinal manifestations of neurogenic dysfunction and bowel dysmotility, as well as malabsorption. [2]
Treatment and therapeutic strategies
Important treatment advances for SSc would entail the development of therapeutic strategies that limit the progression of fibrosis without adversely affecting the overall repair process. [10] To date, there are no entirely effective antifibrotic therapies available for patients with SSc. [15] Penicillamine, methotrexate, photopheresis, relaxin, interferons, and cyclosporine have each been studied in clinical trials with variable response rates. [16] In targeting the role of B cell autoantibodies, recent studies have evaluated treatment with rituximab, an autoantibody directed against the CD20 protein found on the surface of mature B-cells. [3] One study showed that treatment with rituximab may improve lung function in SSc patients, evident by improved forced vital capacity. They also demonstrated improved skin scores in patients treated with rituximab. [17] Various trials have shown conflicting results regarding skin disease and pulmonary function improvement with rituximab, and larger trials are necessary. Imatinib, a tyrosine kinase inhibitor, has recently been considered a potential target for SSc therapy, with case reports showing positive outcomes. The rationale for clinical improvement is that imatinib can block the PDGF and TGFβ signaling pathways. Imatinib exhibits dual antifibrotic effects by inhibition of c-Abl, which is important for activation of TGFβ induced factors, and of the PDGF receptor, which is itself a tyrosine kinase receptor. [15] However, this data is limited by the uncontrolled study designs, and true insight into the efficacy of imatinib treatment relies on placebo-controlled, large-scale studies. [18]
Targeting growth factors involved in the TGF β autocrine loop represents another possible treatment option. CTGF is a potential target of interest, as well as ET-1. An oral prostacyclin, iloprost, has been shown to have in vivo antifibrotic activity via down-regulation of CTGF expression. [2] Bosentan, an endothelin receptor antagonist that blocks the binding of ET-1, has been shown to have success in treating PAH associated with SSc.[3] One recent study in the literature mentions urotensin II as elevated in SSc patients when compared to controls, and correlated to ET-1 levels 19. Urotensin II is a vasoactive peptide with profibrotic features and thought to play a role in the pathogenesis of SSc. Further research is required in order to establish the efficacy of this peptide, however, it might represent another target for treatment options.
There are currently no guidelines in place for the treatment of SSc related vascular disease. Therapy relies on options to alleviate symptoms by addressing the vasospasm, vasculopathy with luminal occlusion, and thrombosis occurring with SSc vascular disease. Despite the evidence of platelet activation and the release of prothrombotic growth factors in SSc, antiplatelet therapy has not shown conclusiveness in controlled trials. [2] On the other hand, calcium channel blockers are being used in the treatment of Raynaud’s phenomenon and digital ischemia. The dihydropyridine calcium channel blockers are potent vasodilators, with additional antioxidant, antithrombotic, and antiapoptotic effects. Clinical trials have shown some benefit in managing Raynaud’s phenomenon with a serotonin-2 receptor antagonist, ketanserin, as the neurotransmitter serotonin is a selective vasoconstrictor. Prostaglandins, which are potent vasodilators, have shown efficacy as well, seen particularly in pulmonary vascular disease and PAH. Prostacyclin, another vasodilator, has protective effects on the endothelium due to its antiproliferative effect on smooth muscle cells, and inhibition of platelet aggregation. [2]
Phosphodiesterases inactivate the second messengers, cAMP, and cGMP for both prostacyclin and nitric oxide, respectively. Phosphodiesterase inhibitors thus prolong and enhance the effects of both the abovementioned vasodilators. Phosphodiesterase-5 inhibitors sildenafil and tadalafil have been shown associated with reduced plasma ET-1 levels, as well as improved capillary flow velocity in patients with Raynaud’s phenomenon. [2] Nitrates represent another possible therapeutic option but are not free of limitations. One laboratory study showed that intra-arterial infusion of nitroprusside or L-arginine (the substrate for NO) decreased cold-induced vasospasm in SSc patients with Raynaud’s phenomenon. However, excessive levels of NO pose the issue of furthering tissue damage by adding to oxidative stress. [2] Few studies have addressed the role of antioxidants in SSc, however, since lipoproteins such as LDL have been shown to contribute to oxidative stress, treatment with cholesterol-lowering medications might provide benefit for scleroderma patients via reduction of oxidative damage.
Severe cases of Raynaud’s phenomenon which are refractory to medical management have shown some improvement with selective digital sympathectomy. The procedure is done under general anesthesia, where vascular innervations visualized under a microscope are physically separated from the blood vessels. Botulinum-toxin injections have a role in nonsurgical sympathectomy, by targeting neurovascular bundles that supply involved digits. Significant pain reduction and improved healing of digital ulcers have been reportedly observed in SSc patients. [20] Reversal of some of the vasculogenic and angiogenic defects seen in SSc, particularly in cases with severe digital ischemia, has been reported with autologous stem cell transplantation. Hematopoietic stem cell transplantation provides new sources of mesenchymal stem cells and progenitor cells and alters the cytokine milieu, leading to an overall recovery of the vascular network, restoration of blood flow, and decreased skin necrosis. [21]
A treatment strategy that has not yet been reported in the literature involves targeting the SMAD family of proteins, specifically by increasing SMAD7 in scleroderma patients. It has been documented that SSc patients have decreased SMAD7, and it is well known that SMAD7 is an inhibitor of SMAD3. Investigators have reported plasmid gene transfer of SMAD7 among knockout mice, demonstrating a correlation between the degree of SMAD7 expression and both fibroblast proliferation and collagen accumulation resulting from cellular responsiveness to TGFβ. [22] A therapeutic strategy involving plasmid transfection and increased expression of SMAD7 represents a newly proposed target pathway for the treatment of SSc. Despite the crucial involvement of TGFβ in regulating fibrosis, inhibition of its autocrine loop may consequently lead to numerous unwanted effects given its role in additional immune functions. A study involving knockout mice with TGFβ null mutants showed death within 3-4 weeks from a rapid wasting syndrome with a widespread inflammatory reaction. [23] Thus, potential therapeutic options aiming to inhibit TGFβ must be approached with caution, and should avoid complete abolition of its function. Nevertheless, a dual-acting therapeutic regimen that increases SMAD7 expression as well as blocks SMAD3 (potentially via receptor antagonism) could hinder the TGFβ signaling pathway, theoretically counteracting the intrinsic defect in SSc tissue fibroblasts.
TREATMENT:
The patient was transferred to another facility for further workup and management. Treatment was initiated with colchicine, topical clobetasol/vitamin D therapy, Lipitor, lisinopril and nifedipine. The patient was also referred to as rheumatology, gastroenterology, and pulmonology. After missing several appointments, her treatment with methotrexate and prednisone had to be deferred. Unfortunately, patient was lost to follow-up.
Systemic sclerosis is a multifaceted autoimmune disorder, of which the etiology remains to be fully understood. Several concepts and theories have been studied to explain the pathogenesis of disease, and it is found to be attributed to a pronounced vasculopathy and endothelial cell damage, leading to microvascular and macrovascular alterations. There is accompanying vast autoimmune dysregulation which furthers vascular disruption. Both immunological and vascular aberrancies contribute to the severe, progressive cutaneous and visceral fibrosis characteristic of SSc. Cutaneous fibrosis and internal organ dysfunction represent a major cause of morbidity and mortality among scleroderma patients. Curative treatment options for this disease do not yet exist, however, increasing understanding of the pathogenesis of SSc will allow for further elucidation of potential therapeutic targets, and the possibility for future treatment success.
REFERENCES:
Gabrielli, A., Avvedimento, E. V., & Krieg, T. (2009). Scleroderma. N Engl J Med, 360(19), 1989-2003. doi:10.1056/NEJMra0800385. PMID: 19420320
Wigley, F. M. (2009). Vascular disease in scleroderma. Clin Rev Allergy Immunol, 36(2-3), 150-175. doi:10.1007/s12016-009-8152-4. PMID: 19107543
Katsumoto, T. R., Whitfield, M. L., & Connolly, M. K. (2011). The pathogenesis of systemic sclerosis. Annu Rev Pathol, 6, 509-537. doi:10.1146/annurev-pathol-011110-130319. PMID: 21166531
Lambova, S. N., & Kuzmanova, S. I. (2006). Raynaud’s phenomenon in common rheumatic diseases. Folia Med (Plovdiv), 48(3-4), 22-28. PMID: 17429468
Derk, C. T., & Jimenez, S. A. (2003). Systemic sclerosis: current views of its pathogenesis. Autoimmun Rev, 2(4), 181-191. doi:10.1016/S1568-9972(03)00019-4. PMID: 12809796
Kelly, B. C., Markle, L. S., Vickers, J. L., et al. (2010). The imbalanced expression of matrix metalloproteinases in nephrogenic systemic fibrosis. J Am Acad Dermatol, 63(3), 483-489. doi:10.1016/j.jaad.2009.09.032. PMID: 20307867
Arnson, Y., Amital, H., Guiducci, S., et al. (2009). The role of infections in the immunopathogenesis of systemic sclerosis–evidence from serological studies. Ann N Y Acad Sci, 1173, 627-632. doi:10.1111/j.1749-6632.2009.04912.x. PMID: 19712006
Wipff, J., Dieude, P., Guedj, M., et al. (2010). Association of a KCNA5 gene polymorphism with systemic sclerosis-associated pulmonary arterial hypertension in the European Caucasian population. Arthritis Rheum, 62(10), 3093-3100. doi:10.1002/art.27615. PMID: 20131290
Agarwal, S. K. (2010). The genetics of systemic sclerosis. Discov Med, 10(51), 134-143. PMID: 20720291
Wynn, T. A. (2008). Cellular and molecular mechanisms of fibrosis. J Pathol, 214(2), 199-210. doi:10.1002/path.2348. PMID: 18161745
Del Papa, N., Quirici, N., Scavullo, C., et al. (2010). Antiendothelial cell antibodies induce apoptosis of bone marrow endothelial progenitors in systemic sclerosis. J Rheumatol, 37(10), 2053-2063. PMID: 20668127
Herrick, A. L., Rieley, F., Schofield, D., et al. (1994). Micronutrient antioxidant status in patients with primary Raynaud’s phenomenon and systemic sclerosis. J Rheumatol, 21(8), 1477-1483. PMID: 8069321
Lundberg, A. C., Akesson, A., & Akesson, B. (1992). Dietary intake and nutritional status in patients with systemic sclerosis. Ann Rheum Dis, 51(10), 1143-1148. doi:10.1136/ard.51.10.1143. PMID: 1467829
Volpe, A., Biasi, D., Caramaschi, P., et al. (2006). Levels of F2-isoprostanes in systemic sclerosis: correlation with clinical features. Rheumatology (Oxford), 45(3), 314-320. doi:10.1093/rheumatology/kei211. PMID: 16172086
Iwamoto, N., Distler, J. H., & Distler, O. (2011). Tyrosine kinase inhibitors in the treatment of systemic sclerosis: from animal models to clinical trials. Curr Rheumatol Rep, 13(1), 21-27. doi:10.1007/s11926-010-0140-4. PMID: 21108517
Habif, T. P. (2010). Clinical Dermatology: A Color Guide to Diagnosis and Therapy (5th ed.). Mosby Elsevier.
Daoussis, D., Liossis, S. N., Tsamandas, A. C., et al. (2010). Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology (Oxford), 49(2), 271-280. doi:10.1093/rheumatology/kep376. PMID: 20005105
Gordon, J., & Spiera, R. (2011). Imatinib and the treatment of fibrosis: recent trials and tribulations. Curr Rheumatol Rep, 13(1), 51-58. doi:10.1007/s11926-010-0157-8. PMID: 21187930
Pehlivan, Y., Onat, A. M., Comez, G., & Babacan, T. (2011). Urotensin-II in systemic sclerosis: a new peptide in pathogenesis. Clin Rheumatol. doi:10.1007/s10067-011-1784-6. PMID: 22003072
Olsen, N. J. (2011). Scleroderma: The Need for Extreme Remedies. Am J Med Sci. doi:10.1097/MAJ.0b013e318219ca1a. PMID: 22039161
Guiducci, S., Porta, F., Saccardi, R., et al. (2010). Autologous mesenchymal stem cells foster revascularization of ischemic limbs in systemic sclerosis: a case report. Ann Intern Med, 153(10), 650-654. doi:10.7326/0003-4819-153-10-201011160-00234. PMID: 21041540
Dong, C., Zhu, S., Wang, T., et al. (2002). Deficient Smad7 expression: a putative molecular defect in scleroderma. Proc Natl Acad Sci U S A, 99(6), 3908-3913. doi:10.1073/pnas.052033199. PMID: 11959987
Kulkarni, A. B., Huh, C. G., Becker, D., et al. (1993). Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A, 90(2), 770-774. doi:10.1073/pnas.90.2.770. PMID: 8421715