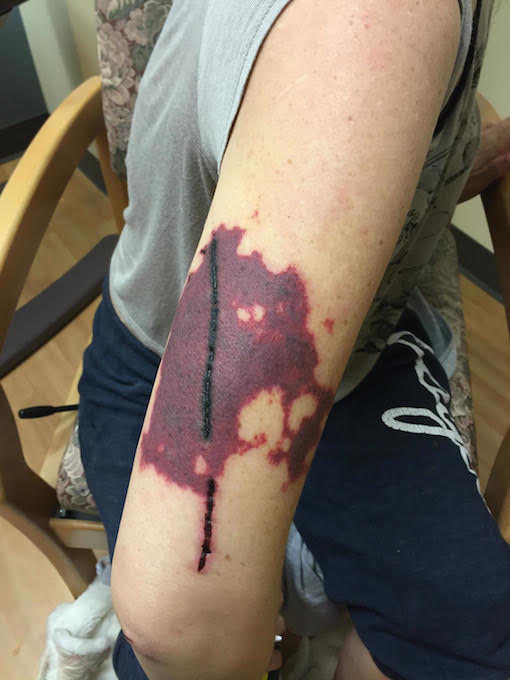
CORRECT DIAGNOSIS:
Drug-Induced SCLE
DISCUSSION:
SCLE is a subtype of the well-known inflammatory autoimmune disorder systemic lupus erythematosus. Both SCLE and systemic lupus erythematosus can be induced by medications. Drug-induced systemic lupus erythematosus differs from drug-induced SCLE in several ways. First of all, drug-induced systemic lupus erythematosus involves diffuse systemic disease usually without a cutaneous presentation, while SCLE always presents with cutaneous involvement. While both forms may involve systemic effects such as serositis and arthralgias, the effects tend to be much more severe in drug-induced systemic lupus erythematosus than in SCLE. Furthermore, the pharmaceutical agents that initiate these two entities differ. While drug-induced systemic lupus erythematosus is often caused by minocycline, hydralazine, and procinamide; SCLE is most often linked to hydrochlorothiazide, calcium channel blockers, ACE-inhibitors, and some antimicrobials. Both SCLE and systemic lupus erythematosus are more common in women and the average age of onset of SCLE is estimated to be 43 years.
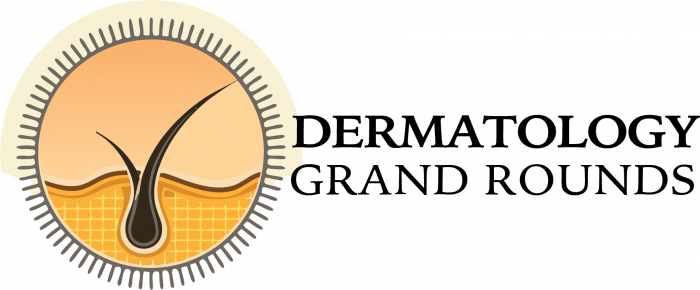
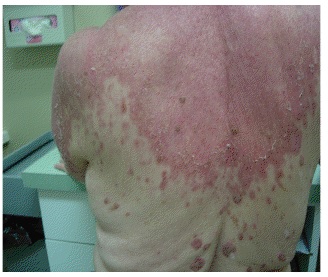
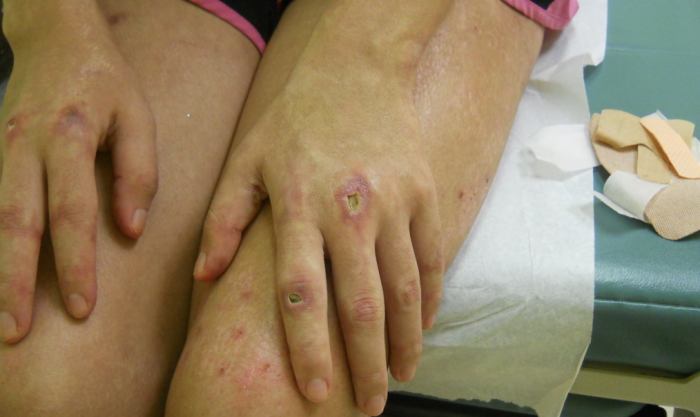
SCLE is characterized by annular, scaly, erythematous papules and plaques that usually present in a photodistributed pattern. The initial papules start out small and later coalesce into larger plaques. The distribution of the rashes follow the typical photosensitivity pattern, which involve the upper back, shoulders, chest, neck, lateral face, and extensor surfaces of the arms. After cessation of the offending agent, the lesions of SCLE usually resolve without scarring. It is possible, however, to develop vitiligo-like patches and/or telangiectasias where the original lesions were. Typical associated symptoms are fever, arthralgias, and myalgias, and it is estimated that 10-15 percent of patients may develop more serious internal manifestations such as nephritis, systemic vasculitis, or elevated liver enzymes as seen in our patient.
SCLE was first described in 1979 by Sontheimer and Thomas et al.; and drug-induced SCLE was first recognized in 1985 secondary to hydrochlorothiazide use. Idiopathic SCLE is a variant of SCLE that is often difficult to differentiate from the drug-induced form. Both serological profiles are similar; however, it has been suggested that the distribution of the lesions may be the only distinguishable factor. Idiopathic SCLE is generally only seen above the waist, while it is common for drug-induced SCLE to be located on the lower extremities, as seen in our patient. The development of SCLE has been noted with a variety of drugs, hydrochlorothiazide is the most common. Other well-known initiators are statins, calcium channel blockers, ACE-inhibitors, and a variety of antimicrobials.8 After initiation of the drug, eruptions usually occur within four to seven weeks.
When evaluating a patient with suspected SCLE, laboratory values are an essential diagnostic tool. Characteristic markers include high ANA titers, Anti-Ro (SS-A) and Anti-La (SS-B) antibodies, which are strongly associated with SCLE. In addition, anti-dsDNA, anti-ssDNA, and anti-histone levels should be drawn. While anti-histone is an identifiable feature of drug-induced systemic lupus erythematosus, it is not pathognomonic for drug-induced SCLE. Some patients express it while some do not. Reports vary as to its actual significance in diagnosis. A report by Bonsmann et al. followed five patients with terbinafine-induced SCLE, all of whom where anti-histone positive. Other case reports, such as one by McKellar et al., describe patients with SCLE who are negative for anti-histone. McKellar presents the case of a middle-aged male with a history of systemic lupus erythematosus who developed drug-induced SCLE after treatment with terbinafine. In this case, the typical anti-Ro (SS-A) antibody was positive, but anti-histone and ANA titers were both negative. The presence of anti-histone antibodies may be beneficial in distinguishing drug-induced SCLE from idiopathic SCLE, as it is not reported to be positive in the idiopathic form. Nuclear immunoflorescense should also be evaluated, and on skin biopsy, SCLE usually presents in a homogenous pattern. After cessation of the offending agent, levels of serum antibodies may be positive for some time. Anti-Ro has been reported to remain positive for up to three years,10 and Anti-La may stay elevated for up to one year. ANA titers typically return to normal limits by six or seven months. There is also a positive correlation between SCLE and the presence of HLA-DR2 and HLA-DR3.
Treatment for patients with drug-induced SCLE initially begins with cessation of the offending agent. Oral and topical steroids are used initially to control the inflammatory response. It is also imperative that sunscreens be used, and avoidance of direct sunlight is highly recommended until there is resolution of the lesions.
In 1998, the first reported case of terbinafine-induced SCLE was described. Since that time, multiple reports have confirmed the link between terbinafine and SCLE. Terbinafine, also known as Lamisil, is an allylamine antifungal agent used commonly in the treatment of fungal infections of the skin and nails. Generally, it is considered to be well tolerated, with an estimated 11% of the patient population experiencing side effects, and 2.7% experiencing skin reactions. The adverse cutaneous reactions range from relatively benign pruritus to life-threatening Stevens-Johnson syndrome and toxic epidermal necrolysis. Terbinafine has even rarely been reported to cause Sweet’s syndrome. Other manifestations include fixed drug reactions, erythema annulare centrifugum, plaque and pustular psoriasis, exanthematus pustulosis, and erythema multiforme. Cutaneous manifestations secondary to terbinafine use typically occur three to four weeks after the initial dose is given.
Terbinafine-induced SCLE appears to affect women more than men. It is also evident that patients with a history of SLE, SCLE, or other autoimmune disorders have a predisposition to develop SCLE when receiving terbinafine treatment. A study by Callen and Hughes et al. followed a group of five patients who developed SCLE after commencement of terbinafine therapy for onychomycosis. Of those five, three had a history of SCLE, one had a possible episode of SCLE, and the other developed it de novo. There have been other reports that favor de novo development rather than an autoimmune predisposition. For example, the study by Bonsmann et al. that was previously mentioned, followed a group of four patients with terbinafine therapy related SCLE, and of this group, only one had a history of possible autoimmunity with Raynauds phenomenon. None of the patients had ever had an episode of SCLE, SLE, or cutaneous photosensitivity.
The pathogenesis by which terbinafine induces SCLE is not known, however it is thought that it may deposit within the keratinocytes leading to autoantibody formation that is seen in SCLE. It is therefore probable that those patients with a known history of autoimmunity may be more susceptible to developing terbinafine induced SCLE. In addition, anti-Ro has been linked to causing cytotoxic damage to keratinocytes that is seen in lupus.
In conclusion, drug-induced SCLE is a condition that tends to occur among patients with a history of autoimmune skin conditions. There are a multitude of medications that can induce the cutaneous eruptions seen in SCLE. Terbinafine is a rare cause of drug-induced SCLE, but is important to consider due to its wide use in dermatology. While usually safe to use, it should be used with extreme caution in patients with a history of autoimmune disease, specifically SLE, photosensitivity, or elevated ANA titers. It is also advised that patients with a history of positive anti-Ro antibodies avoid using terbinafine unless necessary. Subsequently, cases of dermatophyte infection should be confirmed by nail clippings and culture before initiation of terbinafine therapy in individuals prone to autoimmunity. If SCLE does ensue after treatment, patients should be advised to discontinue the terbinafine and be assured that the skin manifestations are temporary and should resolve when the medication is eliminated. Terbinafine is, however, still considered to be a safe medication, and its use ultimately should depend on the physician’s clinical judgment.
TREATMENT:
A Kenalog intramuscular injection of 40mg was administered. She was also given Atarax 25mg po daily and Prednisone taper of 20 mg three daily for four days, then two pills for four days and then one pill for four days. She also received Triamcinolone 0.5% cream for the body, and Desonide 0.05% cream for the face.
At the one week follow-up appointment, the patient stated that her condition was 90% improved.
REFERENCES:
Vassileva, S., Mateev, G., & Parish, L. C. (1998). Antimicrobial photosensitive reactions. Archives of Internal Medicine, 158(18), 1993-2000. doi:10.1001/archinte.158.18.1993
Callen, J. (2001). Drug-induced cutaneous lupus erythematosus: A distinct syndrome that is frequently unrecognized. Journal of the American Academy of Dermatology, 46(2), 315-316. doi:10.1067/mjd.2002.111343
Callen, J., Hughes, A., & Kulp-Shorten, C. (2001). Subacute cutaneous lupus erythematosus induced or exacerbated by terbinafine. Archives of Dermatology, 137(9), 1196-1198. doi:10.1001/archderm.137.9.1196
Chlebus, E., Wolska, H., Blaszczyk, M., & Jablonska, S. (1998). Subacute cutaneous lupus erythematosus versus systemic lupus erythematosus: Diagnostic criteria and therapeutic implications. Journal of the American Academy of Dermatology, 38(3), 405-412. doi:10.1016/S0190-9622(98)70178-0
Weger, W., Kranke, B., Gerger, A., Salmhofer, W., & Aberer, E. (2008). Occurrence of subacute cutaneous lupus erythematosus after treatment with fluorouracil and capecitabine. Journal of the American Academy of Dermatology, 59(2), S4-S6. doi:10.1016/j.jaad.2008.05.018
Bonsmann, G., Schiller, M., Luger, T., & Stander, S. (2001). Terbinafine-induced subacute cutaneous lupus erythematosus. Journal of the American Academy of Dermatology, 44(6), 926-931. doi:10.1067/mjd.2001.113263
Wolff, K., Goldsmith, L. A., Katz, S. I., Gilchrest, B. A., Paller, A. S., & Leffell, D. J. (2008). Fitzpatrick’s dermatology in general medicine (7th ed.). New York: McGraw-Hill, Inc.
Kuhn, A., Gensch, K., & Bonsmann, G. (2006). Cutaneous lupus erythematosus. Part 1: Clinical manifestations and classifications. Hautarzt, 57, 251-268. doi:10.1007/s00105-006-1070-0
Hall, M., Monka, C., Krupp, P., & O’Sullivan, D. (1997). Safety of oral terbinafine: Results of a post-marketing surveillance study in 25,884 patients. Archives of Dermatology, 133(10), 1213-1219. doi:10.1001/archderm.133.10.1213
Reed, B., Huff, J., Jones, S., Orton, P., Lee, L., & Norris, D. (1985). Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Annals of Internal Medicine, 103, 49-51. doi:10.7326/0003-4819-103-1-49
Crowson, A. N., & Magro, C. M. (1997). Subacute cutaneous lupus erythematosus arising in the setting of calcium channel blocker therapy. Human Pathology, 28, 67-73. doi:10.1016/S0046-8177(97)90249-1
Tabhrner, R., Puig, L., Gilaberte, M., & Alomar, A. (2003). Acute generalized exanthematous pustulosis induced by terbinafine. European Journal of Dermatology, 13(3), 313-314. doi:10.1684/ejd.2003.0043
Belgi, A., Konstantopoulou, M., & Macfarlane, A. (2005). Sweet’s syndrome following oral terbinafine therapy: A case report. Journal of the American Academy of Dermatology, 52(3), P60. doi:10.1016/j.jaad.2004.10.003
Castellsague, J., Garcia-Rodriguez, L. A., Duque, A., & Perez, S. (2002). Risk of serious skin disorders among users of oral antifungals: A population-based study. BMC Dermatology, 2(14). doi:10.1186/1471-5945-2-14
Lombardo, M., Cerati, M., & Pazzaglia, A. (2003). Acute generalized exanthematous pustulosis induced by terbinafine. Journal of the American Academy of Dermatology, 49(1), 158-159. doi:10.1067/mjd.2003.151