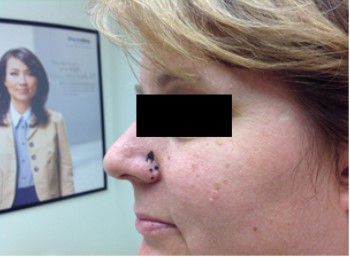
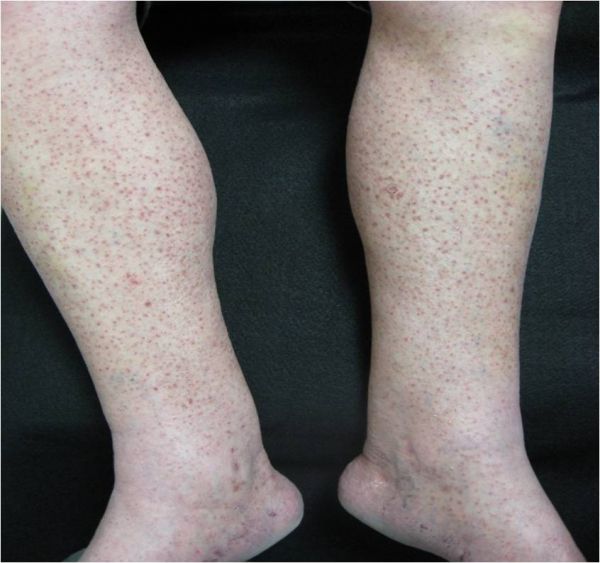
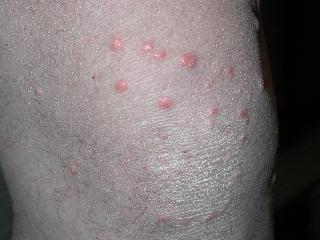
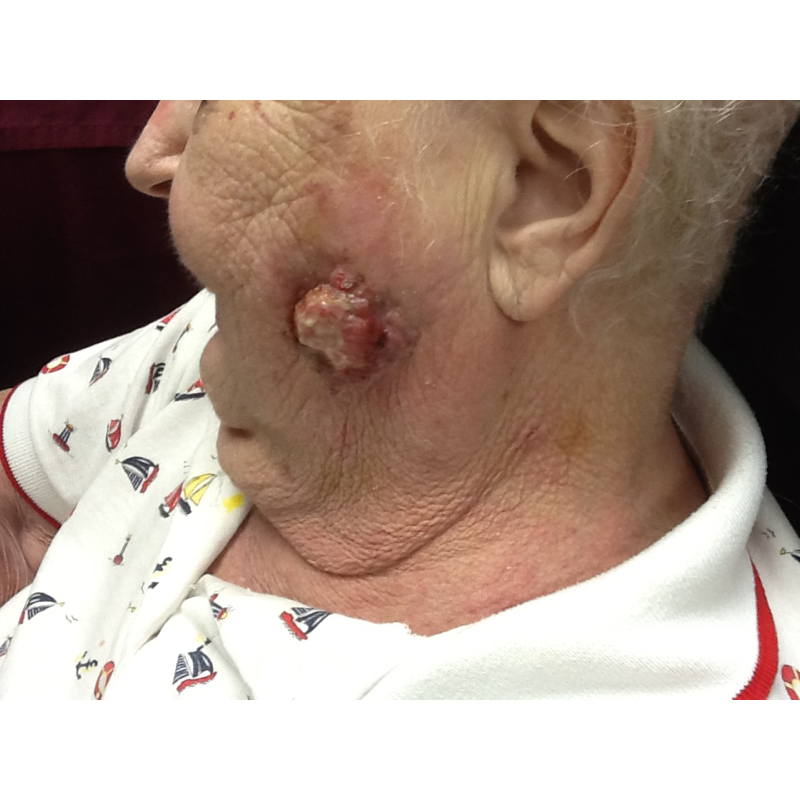
CORRECT DIAGNOSIS:
Sebaceous Epithelioma
DISCUSSION:
Due to this patient’s history of colon cancer and her strong family history of colon cancer, this patient was diagnosed with having Muir- Torre Syndrome (MTS). In this syndrome patients have a genetic predisposition to developing sebaceous neoplasms and visceral malignancies. It represents a variant of Hereditary Nonpolyposis Colorectal Cancer (HNPCC) Syndrome, aka Lynch syndrome, where patients develop mutations in mismatch repair genes. There are two types of MTS. Type 1 is 65% more common, is inherited in an autosomal dominant fashion, and has mutations in MSH2, MLH1, MSH6, and PMS2. These patients develop earlier onset tumors. MTS type 2 occurs in 35 % of patients, is autosomal recessive, and does not have microsatellite instability. They have a mutation in MYH1 and have later onset of tumors.
Sebaceous neoplasms are seen in patients with MTS and rarely in patients without MTS. Sebaceous neoplasms includes adenomas, epitheliomas, and carcinomas. They may occur outside of the head and neck region and are described as painless, slow growing, pink or yellow papules, plaques, or nodules often with central umbilication and ulceration.
A known association with MTS is the finding of multiple keratoacanthomas. Gastrointestinal cancers are found in 61%, specifically colorectal adenocarcinoma. Genitourinary caners are found in 22% of patients, and approximately 15% of female patients develop endometrial cancer. There are many other types of cancers associated with this syndrome as well.
In order to diagnosis MTS there must be one sebaceous neoplasm and at least one internal organ cancer present. Immunohistochemical testing can be performed on the tumor which will show a loss of staining of the genes if MTS is present. The sensitivity if this test is 81% and the PPV is 31%,1 however there are varying results in papers. Currently there are debates on whether or not dermatopathologists should screen all sebaceous neoplasm with IHC for MTS.2 Our patient did not undergo testing due to her personal history and strong family history of colon cancer. If a patient has multiple keratoacanthomas, one should consider IHC testing. Microsatellite instability gene analysis can also be performed on the tumor where instability in 2 of 5 markers indicates a high probability of MTS. Sebaceous carcinoma found near the orbit warrants a lymph node evaluation, however if it is located outside of the orbit it is not routinely recommended. Patients are recommended to get upper and lower GI endoscopies; genitourinary surveillance that incudes prostate, testicle, breast, and pelvis exams; endometrial biopsies; and cervical and urine cytology. They are also recommended to get a chest x-ray, CEA level, fecal occult blood testing, serial hemoglobin levels, and LFT’s.
TREATMENT:
The recommended treatment for sebaceous neoplasms is wide local excision with 5-6 mm margins. Mohs is appropriate for sebaceous carcinomas. Radiation can be used as an adjunct to excision if there is recurrence, regional metastasis, or if it is for palliative treatment. Genetic counseling should be used for the patient and family members. Our patient is currently undergoing genetic testing and we are awaiting the results.
REFERENCES:
Jessup, C. J., Redston, M., Tilton, E., & Reimann, J. D. (2016). Importance of universal mismatch repair protein immunohistochemistry in patients with sebaceous neoplasia as an initial screening tool for Muir-Torre syndrome. Human Pathology, 49, 1-9. https://doi.org/10.1016/j.humpath.2016.01.006 [PMID: 26794303]
Kim, R. H., Nagler, A. R., & Meehan, S. A. (2016). Universal immunohistochemical screening of sebaceous neoplasms for Muir-Torre syndrome: Putting the cart before the horse? Journal of the American Academy of Dermatology, 75(5), 1078-1079. https://doi.org/10.1016/j.jaad.2016.06.015 [PMID: 27592034]
John, A. M., & Schwartz, R. A. (2016). Muir-Torre syndrome (MTS): An update and approach to diagnosis and management. Journal of the American Academy of Dermatology, 74(3), 558-566. https://doi.org/10.1016/j.jaad.2015.10.045 [PMID: 26709102]