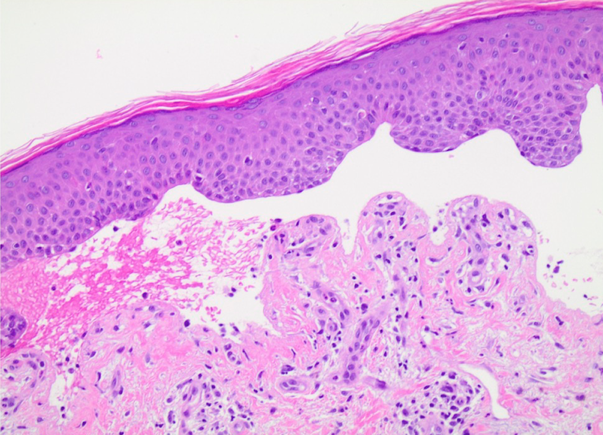
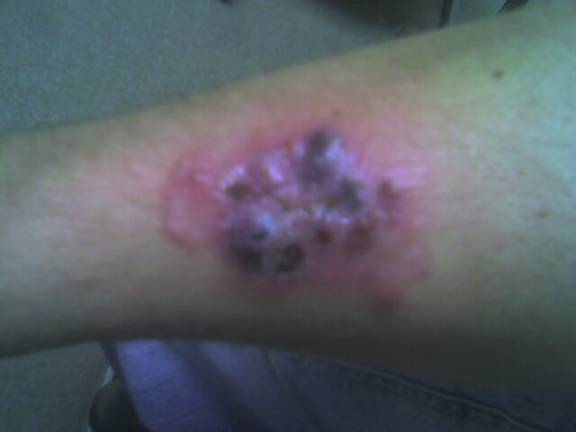
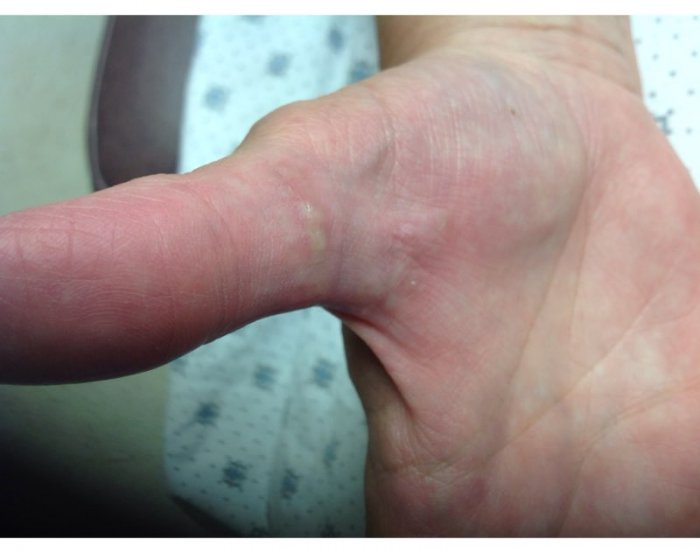
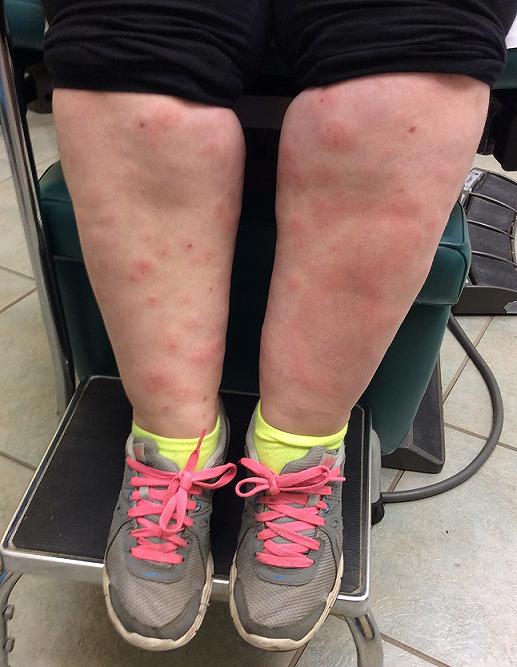
CORRECT DIAGNOSIS:
Nivolumab induced bullous pemphigoid
DISCUSSION:
Autoimmune bullous dermatoses are a recently described adverse event to the PD-1/PD-L1 inhibitors.1 The pathogenesis of BP may be due to excessive B-cell costimulation and increased autoantibody production with blockade of PD-1/PD-L1 receptors.1,2,3 None of the prior patients mentioned neurologic or cutaneous metastases, which potentially could have an effect on exposing epitopes for the development of BP. A diagnosis of lichen planus pemphigoides instead of BP is another consideration given that BP180 is elevated in lichen planus pemphigoides and lichenoid eruptions are a common cutaneous adverse effect to immune checkpoint inhibitors. However, none of the patients had coexisting lichen planus or a lichenoid infiltrate on pathology. Given that all the patients have been elderly and the majority displayed the characteristic distribution of BP, it is possible that some of the patients previously could have had low titer autoantibodies, yet clinical significance of their disease was precipitated by the PD-1/PD-L1 inhibitors.
Of the reported cases of PD-1/PD-L1 inhibitor-induced BP, the majority were controlled with topical and/or oral steroids alone.1,2 This is a therapeutic challenge due to the necessity of balancing the effects of immunosuppressive medications in a patient with severe BP, who was previously successful on nivolumab and without alternative options. In our patient, plasma exchange was chosen because it has targeted immunosuppressive effects, has a rapid onset of action, and has been used successfully to treat BP.4 Rituximab alone has previously been used successfully in treating and preventing relapse of nivolumab-induced BP6; however, after multiple hospitalizations, an adjunctive rapid treatment modality was also needed. Interestingly, recent evidence suggests that some melanomas have subpopulations of CD20-positive “tumor stem cells” that respond to anti-CD20 therapy.5 Therefore, rituximab itself may have potential benefits in this population. This case is novel because it demonstrates the potential for rapid and sustained improvement in nivolumab-induced BP with plasma exchange and rituximab for targeted immunomodulation in the increasing population of patients presenting with unique autoimmune phenomenon with checkpoint inhibition.
TREATMENT:
Consequently, plasma exchange was started. He completed 5 sessions of plasma exchange, immediately followed by two 1000 mg rituximab infusions 15 days apart, which resulted in near clearing (Figure 1B). He continues to use betamethasone diproprionate 0.05% cream twice daily without systemic corticosteroids.
REFERENCES:
1. Naidoo J, Schindler K, Querfeld C, et al. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunology Research. 2016;4(5):383-389. doi:10.1158/2326-6066.cir-15-0123.
2. Damsky W, Kole L, Tomayko MM. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Reports. 2016;2(6):442-444. doi:10.1016/j.jdcr.2016.05.009.
3. Hwang SJ, Carlos G, Chou S, Wakade D, Carlino MS, Fernandez-Penas P. Bullous pemphigoid, an autoantibody-mediated disease, is a novel immune-related adverse event in patients treated with anti-programmed cell death 1 antibodies. Melanoma Research. 2016;26(4):413-416. doi:10.1097/cmr.0000000000000260.
4. Mazzi G, Raineri A, Zanolli FA, et al. Plasmapheresis therapy in pemphigus vulgaris and bullous pemphigoid. Transfusion and Apheresis Science. 2003;28(1):13-18. doi:10.1016/s1473-0502(02)00095-2.
5. Pinc A, Somasundaram R, Wagner C, et al. Targeting CD20 in Melanoma Patients at High Risk of Disease Recurrence. Molecular Therapy. 2012;20(5):1056-1062. doi:10.1038/mt.2012.27.
6. Sowerby L, Dewan A, Granter S, Gandhi L, LeBoeuf N. Rituximab treatment of nivolumab-induced bullous pemphigoid. JAMA Dermatology Letters. 2017.